
Whey kan bekämpa fetma och diabetes typ-2
Whey Protein ger stora metabola effekter
I en nyligen publicerad rapport med det långa namnet "Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes" visas att det metabola syndromet med övervikt och diabetes typ-2 förefaller kunna mildras i symtomatologi och omfattning via en regelbunden konsumtion av Whey protein. Effektvägarna är flera, från att öka mättnadskänslan, öka termogenesis d.v.s. kroppens produktion av värme samt via en rad andra potentieringar av molekylära funktionsvägar exempelvis så visar Proteinet Whey(vassleprotein) upp en glukossänkande och insulinotropisk effekt samt påverkar det kraftfulla reglersystemet
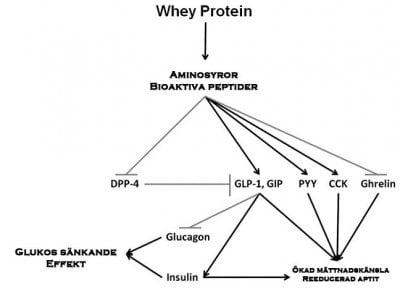
Konsumtionen av mjölk och mejeriprodukter har förknippats med minskad risk för metabola sjukdomar och hjärt-kärlsjukdomar. Mjölk innehåller två primära proteinkällor, kasein (80 %) och Whey (vassle) (20 %). Nyligen har de gynnsamma fysiologiska effekter av vassleprotein på kontroll av födointag och glukosmetabolismen rapporterats. Studier har visat en insulinotropisk och glukossänkande egenskaper wheyprotein hos friska och typ 2-diabetes patienter. Wheyprotein verkar inducera dessa effekter via bioaktiva peptider och aminosyror som genereras under dess magmatsmältning. Dessa aminosyror och peptider stimulerar frisättningen av flera tarmhormoner såsom kolecystokinin, peptid YY och inkretiner gastrisk hämmande peptid och glukagon-liknande peptid 1 som förstärker insulinutsöndring från β-celler och är förknippade med regleringen av födointag. De bioaktiva peptider genereras från vassleprotein kan också tjäna som endogena hämmare av dipeptidylpeptidas-4 (DPP-4) i den proximala tarmen och förhindrar inkretiner nedbrytning. Faktum nyligen var DPP-4-hämmare identifieras i hydrolysat vassleprotein. Denna översyn kommer att fokusera på de nya egenskaper whey-protein och dess potentiella kliniska effekter vid fetma och typ 2-diabetes.
Whey (Vassleprotein) Slutsats
Whey Vassleprotein utöver sin effekt via via bioaktiva peptider och aminosyror som genereras i magen under matsmältning och ökar frisättningen av flera hormoner, såsom CCK, PYY, GIP, GLP-1 och insulin vilka leder till minskat födointag och ökad mättnad (fig. 1). Insulin sekretion är associerad med glukossänkande effekt och med kontroll av födointag. Den mekanism genom vilken vassleproteiner leder till ökad insulinutsöndring är för närvarande inte känd och bör undersökas. En möjlig mekanism är produktionen av bioaktiva peptider som fungerar som endogena inhibitorer av DPP-4 i den proximala tarmen, förhindra nedbrytningen av den insulinotropiska inkretiner GLP-1 och GIP. En annan mekanism kan vara BCAA, speciellt leucin, vilket aktiverar mTOR signalväg och proteinsyntes vilket leder till förhöjda hormon uttryck och sekretion samt en ökad termogenes. Den insulinotropiska effekten av vassleproteiner kan potentiellt dämpa postprandiala variationerna i blodglukosvärdet över dagen och kan därmed förbättra glukoshomeostas i typ 2-diabetes och kan möjligen skjuta upp införandet av medicinsk behandling. Förmågan att amplifiera insulinutsöndring genom vassleprotein kan vara säkrare än de vanligen använda terapeutiska medlen.
Wheyproteinet ökade på mättnadskänsla såväl som termogenes och visade dessutom fleraförändringar i blodsockerbilden glukoshomeostasen fullt jämförbara med en läkemedelsbehandling vilket stödjer tillämpningen av vassleprotein i den terapeutiska behandlingen av typ 2-diabetes och fetma. Icke desto mindre bör framtida studier avgöra om dessa gynnsamma effekter av vassleprotein på födointag och de subjektiva upplevelserna av mättnadkänsla efter intag hos människa också erhålls också vid ett långvarigt bruk med en daglig konsumtion av vassleprotein.
Förkortningar: GMP, glykomakropeptid, DPP-4, dipeptidylpeptidas-4, GIP, glukosberoende insulinotropisk polypeptid, GLP-1, glukagon-liknande peptid-1, BCAA, grenade aminosyror, PYY, peptid YY, CCK, kolecystokinin
Nyckelord: Vassle, diabetes typ-2, fetma, GLP-1, DPP-4, mjölk, Obesitas, Metabolt syndrom
Refererande studie om Whey och Diabetes:
Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes.
J Nutr Biochem. 2013 Jan;24(1):1-5. doi: 10.1016 j.jnutbio.2012.07.008.
Jakubowicz D, Froy O. Source Diabetes Unit E. Wolfson Medical Center, Tel Aviv University, Holon 58100, Israel. Electronic address: [email protected].
Studiens referenser
Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604
Vander Wal JS, Gupta A, Khosla P, Dhurandhar NV. Egg breakfast enhances weight loss. Int J Obes (Lond). 2008;32:1545–1551
Jakubowicz D, Froy O, Wainstein J, Boaz M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids. 2012;77:323–331
Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, et al. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr. 2009;139:514–521
Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385
Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41
Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–248
Tremblay A, Gilbert JA. Milk products, insulin resistance syndrome and type 2 diabetes. J Am Coll Nutr. 2009;28(Suppl 1):91S–102S
Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr. 2007;26:713S–723S
Madureira AR, Tavares T, Gomes AM, Pintado ME, Malcata FX. Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci. 2010;93:437–455
Luhovyy BL, Akhavan T, Anderson GH. Whey proteins in the regulation of food intake and satiety. J Am Coll Nutr. 2007;26:704S–712S
Satake M, Enjoh M, Nakamura Y, Takano T, Kawamura Y, Arai S, et al. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biosci Biotechnol Biochem. 2002;66:378–384
Tappy L, Jequier E, Acheson K. Thermic effect of infused amino acids in healthy humans and in subjects with insulin resistance. Am J Clin Nutr. 1993;57:912–916
Acheson KJ, Ravussin E, Wahren J, Jequier E. Thermic effect of glucose in man. Obligatory and facultative thermogenesis. J Clin Invest. 1984;74:1572–1580
Acheson KJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Emady-Azar S, Ammon-Zufferey C, et al. Protein choices targeting thermogenesis and metabolism. Am J Clin Nutr. 2011;93:525–534
Robinson SM, Jaccard C, Persaud C, Jackson AA, Jequier E, Schutz Y. Protein turnover and thermogenesis in response to high-protein and high-carbohydrate feeding in men. Am J Clin Nutr. 1990;52:72–80
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–14935
Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–323S
Appuhamy JA, Knoebel NA, Nayananjalie WA, Escobar J, Hanigan MD. Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J Nutr. 2012;142:484–491
Li F, Yin Y, Tan B, Kong X, Wu G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41:1185–1193
Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci. 2007;85:919–927
Pruznak AM, Kazi AA, Frost RA, Vary TC, Lang CH. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribonucleoside prevents leucine-stimulated protein synthesis in rat skeletal muscle. J Nutr. 2008;138:1887–1894
Mahe S, Roos N, Benamouzig R, Davin L, Luengo C, Gagnon L, et al. Gastrojejunal kinetics and the digestion of [15N]beta-lactoglobulin and casein in humans: the influence of the nature and quantity of the protein. Am J Clin Nutr. 1996;63:546–552
Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, et al. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr. 2007;86:1364–1368
Liljeberg Elmstahl H, Bjorck I. Milk as a supplement to mixed meals may elevate postprandial insulinaemia. Eur J Clin Nutr. 2001;55:994–999
Pal S, Ellis V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br J Nutr. 2010;104:1241–1248
Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V. A whey protein supplement decreases post-prandial glycemia. Nutr J. 2009;8:47
Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr. 2010;91:966–975
Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr. 2010;104:716–723
Nilsson M, Holst JJ, Bjorck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr. 2007;85:996–1004
Ma J, Stevens JE, Cukier K, Maddox AF, Wishart JM, Jones KL, et al. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32:1600–1602
Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr. 2005;82:69–75
Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–1502
Gao Z, Young RA, Li G, Najafi H, Buettger C, Sukumvanich SS, et al. Distinguishing features of leucine and alpha-ketoisocaproate sensing in pancreatic beta-cells. Endocrinology. 2003;144:1949–1957
Smith TJ, Stanley CA. Untangling the glutamate dehydrogenase allosteric nightmare. Trends Biochem Sci. 2008;33:557–564
Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68:270–279
Branstrom R, Efendic S, Berggren PO, Larsson O. Direct inhibition of the pancreatic beta-cell ATP-regulated potassium channel by alpha-ketoisocaproate. J Biol Chem. 1998;273:14113–14118
McTaggart JS, Clark RH, Ashcroft FM. The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J Physiol. 2010;588:3201–3209
Yang J, Wong RK, Park M, Wu J, Cook JR, York DA, et al. Leucine regulation of glucokinase and ATP synthase sensitizes glucose-induced insulin secretion in pancreatic beta-cells. Diabetes. 2006;55:193–201
Yang J, Wong RK, Wang X, Moibi J, Hessner MJ, Greene S, et al. Leucine culture reveals that ATP synthase functions as a fuel sensor in pancreatic beta-cells. J Biol Chem. 2004;279:53915–53923
Kazafeos K. Incretin effect: GLP-1, GIP, DPP4. Diabetes Res Clin Pract. 2011;93(Suppl 1):S32–S36
Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr. 2004;43:127–139
Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Bjorck I, et al. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on beta-cells. Nutr Metab (Lond). 2012;9:48
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, et al. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav. 2009;96:675–682
Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in Type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723
Nauck MA, Vilsboll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care. 2009;32(Suppl 2):S223–S231
Brubaker PL, Drucker DJ. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659
Portha B, Tourrel-Cuzin C, Movassat J. Activation of the GLP-1 receptor signalling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res. 2011;376509;in press
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157
Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520
Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830
Gribble FM, Manley SE, Levy JC. Randomized dose ranging study of the reduction of fasting and postprandial glucose in type 2 diabetes by nateglinide (A-4166). Diabetes Care. 2001;24:1221–1225
Kitabchi AE, Kaminska E, Fisher JN, Sherman A, Pitts K, Bush A, et al. Comparative efficacy and potency of long-term therapy with glipizide or glyburide in patients with type 2 diabetes mellitus. Am J Med Sci. 2000;319:143–148
Darmoul D, Voisin T, Couvineau A, Rouyer-Fessard C, Salomon R, Wang Y, et al. Regional expression of epithelial dipeptidyl peptidase IV in the human intestines. Biochem Biophys Res Commun. 1994;203:1224–1229
Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363
Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–878
Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, et al. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology. 2006;147:3173–3180
Tulipano G, Sibilia V, Caroli AM, Cocchi D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides. 2011;32:835–838
Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91:1477–1483
Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr. 2011;141:1489–1494
Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A. Brain amino acid requirements and toxicity: the example of leucine. J Nutr. 2005;135:1531S–1538S
Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–E171
Figlewicz DP, Nadzan AM, Sipols AJ, Green PK, Liddle RA, Porte D, et al. Intraventricular CCK-8 reduces single meal size in the baboon by interaction with type-A CCK receptors. Am J Physiol. 1992;263:R863–R867
le Roux CW, Bloom SR. Peptide YY, appetite and food intake. Proc Nutr Soc. 2005;64:213–216 Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135:1320–1325
Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–2919
Andra inlägg
- Whey Proteinpulver Historia med Vassleprotein
- Whey Proteinpulver Billigt just nu
- Whey Proteinpulver från Vassleprotein Historia
- Whey Protein Proteinpulver Vassleprotein exempel
- Whey Proteinpulver bilder
- Whey Proteinpulver tar rekordet!
- Vassleprotein Whey proteinpulver kan förbättra levervärdena
- Kan Whey Protein hjälpa mig att gå ned i Vikt?
- Vad är bäst Vassle Protein eller Kasein protein?
